

More than 200 Distributors
Around 1,000,000 Outlets
Nationwide Coverage
Toptic
(Tobramycin)
Brand Name
Toptic
Generic Name
(Tobramycin)
Therapeutic Segment
Antibiotic (Aminoglycoside)
Available as
- DROPS
- TOPTIC 0.3% EYE DROPS
PRESCRIBING INFORMATION
TOPTIC Eye Drops is sterile topical preparation containing tobramycin. Tobramycin is a water-soluble aminoglycoside antibiotic active against a wide variety of ophthalmic bacterial pathogens. The chemical structure of tobramycin is represented as:
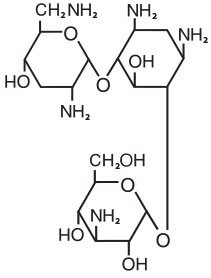
Empirical formula: C18H37N5O9
Molecular weight: 467.5
Chemical name: 4-O-(3-Amino-3-deoxy-α-D-glucopyranosyl)-2-deoxy-6-O-(2,6,-diamino-2,3,6-trideoxy-α-D-ribo-hexo pyranosyl)-L-streptamine
CAS Registry Number: 32986-56-4
DESCRIPTION
TOPTIC Eye Drops have been formulated as a sterile, preserved solution for topical application to the eye. TOPTIC Eye Drops contains 3 mg/mL tobramycin, together with boric acid, sodium sulfate, sodium chloride, tyloxapol, sodium hydroxide and/or sulfuric acid (to adjust pH) and purified water. The solution is preserved with benzalkonium chloride (0.01%).
PHARMACOLOGY ACTIONS
Tobramycin is a water-soluble aminoglycoside antibiotic active against a variety of ophthalmic bacterial pathogens. In vitro studies have shown tobramycin to be active against susceptible strains of the following microorganisms:
Gram-positive:
Staphylococci, including methicillin-susceptible and -resistant Staphylococcus aureus, S. epidermidis, and other coagulase-negative species.
- Streptococcus species including Streptococcus pneumoniae.
Gram-negative:
- Acinetobacter spp.
- Citrobacter spp.
- Enterobacter spp.
- Escherichia coli
- Haemophilus influenzae (beta lactamase-negative and -positive)
- Klebsiella pneumoniae
- Moraxella spp.
- Neisseria meningitidis
- Proteus mirabilis
- Pseudomonas aeruginosa
- Serratia marcescens
Resistance development to the various aminoglycosides such as tobramycin or gentamicin is due to their close structural relationship. Resistance to one aminoglycosides generally confers resistance to several, but not all aminoglycosides. For instance, bacterial susceptibility studies demonstrate that in some cases bacteria resistant to gentamicin retain susceptibility to tobramycin. In vitro results, however, are not necessarily reliable predictors of clinical success in topical therapy.
PHARMACOKINETICS
Tobramycin and other aminoglycoside antibiotics exist in solution as highly polar cations and, therefore, do not readily penetrate external ocular tissues nor are they readily absorbed after oral administration. In rabbits, peak cornea tissue concentrations were similar for both TOPTIC Eye Drops and Eye Ointment at 0.5 to 0.8 mcg/g. In humans, serum concentrations were less than 0.2 mcg/mL during a 36-hour treatment regimen with a 1.5% solution (cumulative topical ocular dose of 50 mg). In comparison, therapeutic intramuscular doses of 1 mg/kg every 8 hours gave peak plasma concentrations of 5 – 8 mcg/mL.
Tobramycin is excreted rapidly and extensively in the urine via glomerular filtration; primarily as unchanged drug. The plasma half-life has been reported to be 2.2 hours. Plasma protein binding is less than 10%.
INDICATIONS
Treatment of external infections of the eye and its adnexa caused by susceptible bacteria. Appropriate monitoring of bacterial response to topical antibiotic therapy should accompany the use of TOPTIC.
CONTRAINDICATIONS
Contraindicated in patients with known hypersensitivity to tobramycin or any other ingredients in this product.
PRECAUTIONS
FOR TOPICAL USE ONLY. NOT FOR INJECTION INTO THE EYE
Hypersensitivity
Sensitivity to topically applied aminoglycosides may occur in some patients. If a sensitivity reaction to TOPTIC Eye Drops occurs, discontinue use. Serious adverse reactions including neurotoxicity, ototoxicity and nephrotoxicity have occurred in patients receiving systemic tobramycin therapy. Although these effects have not been reported following topical ocular use of tobramycin, caution is advised when used concomitantly.
General
As with other antibiotic preparations, prolonged use may result in overgrowth of non-susceptible organisms, including fungi. If super infection occurs, appropriate therapy should be initiated. Cross-sensitivity to other aminoglycoside antibiotics may occur. The possibility that patients that become sensitised to topical ocular tobramycin may also be sensitive to other topical and/or systemic aminoglycosides should be considered.
Ophthalmic solutions and ointments may retard corneal wound healing.
Effects on fertility
Studies have not been performed to evaluate the effect of topical ocular administration of TOPTIC (Tobramycin) Eye Drops on human fertility.
Interactions with other medicines
Concurrent use of topical ocular tobramycin with a topical, ü-lactam type antibiotic may result in inactivation of tobramycin.
If topical ocular tobramycin is administered concomitantly with systemic aminoglycoside antibiotics, the possibility of increased systemic toxicity cannot be excluded and care should be taken to monitor the total serum concentration. Prolonged levels above 12 mcg/mL should be avoided.
Aminoglycosides administered parenterally have been associated with neuromuscular and respiratory paralysis in cats. Despite the low systemic levels of tobramycin associated with ophthalmic administration, pulmonary status of anaesthetised patients should be monitored.
Peak and trough levels of parenterally administered aminoglycosides have been significantly elevated by the concomitant administration of indomethacin.
Concurrent and/or sequential use of TOPTIC with other drugs with neurotoxic or ototoxic potential should be avoided.Antiemetics, such as dimenhydrinate, may mask the toxic effects of drugs causing ototoxicity.
Use In Pregnancy- Category B3
There are no adequate, well-controlled studies using the topical administration of TOPTIC in pregnant women. A published retrospective assessment of women receiving parenteral aminoglycosides during pregnancy suggested no detectable teratogenic risk to the foetus. Studies in animals have shown evidence of an increased occurrence of foetal damage following systemic administration of aminoglycosides to pregnant mothers. There is evidence of selective uptake of aminoglycosides by the foetal kidney resulting in damage (probably reversible) to immature nephrons. Eighth cranial nerve damage has also been reported following in utero exposure to some of the aminoglycosides. Because of their chemical similarity, all aminoglycosides must be considered potentially nephrotoxic and ototoxic to the foetus. It should also be noted that therapeutic blood concentrations in the mother do not equate with safety for the foetus. Use of TOPTIC by pregnant women is not likely to result in detectable blood concentrations in mothers or tissue concentrations in the foetus. TOPTIC should not be used during pregnancy unless clearly needed, otherwise tobramycin is not recommended during pregnancy.
Use In Lactation
In systemic treatment, tobramycin passes over to human milk in such amounts that there is a risk of affecting the child. As systemic absorption when instilled topically in the eye is low, the risk is judged to be low when using TOPTIC but this should be considered when prescribing TOPTIC to nursing mothers. TOPTIC should be used only if the potential benefit for the mother justifies the potential risk to the infant.
Paediatric Use
Safety and effectiveness in children below the age of 1 year have not been established.
Use In Elderly
No overall differences in safety or effectiveness have been observed between elderly and other adult patients.
Renal, Auditory, Vestibular, or Neuromuscular Impairment
Patients receiving concomitant parenteral tobramycin (aminoglycoside) and topical tobramycin therapies should be monitored as clinically appropriate. Caution should be exercised with known or suspected renal, auditory, vestibular, or neuromuscular dysfunction.
Renal & Hepatic Impairment
TOPTIC has not been studied in these patient populations. However, due to low systemic absorption of tobramycin after topical administration of this product, dose adjustment is not necessary.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted to evaluate the carcinogenic potential of tobramycin. In vitro and in vivo studies with tobramycin did not reveal a mutagenic potential.
Contact Lenses
TOPTIC Eye Drops should be instilled while the patient is wearing contact lenses. Contact lens wear is not recommended during treatment of an ocular infection.
If patients continue to wear contact lenses while under treatment with TOPTIC Eye Drops, they should remove their lens(es) prior to instilling the drops in the affected eye(s). Lens(es) should not be inserted into the eye(s) until 15 minutes after instillation of the drops. TOPTIC Eye Drops contains benzalkonium chloride which may cause eye irritation and is known to discolor soft contact lenses. Avoid contact with soft contact lenses.
Effects On Ability to Drive and Use Machines
As with other ophthalmic medications, temporary blurred vision or other visual disturbances may affect the ability to drive or use machines. If blurred vision occurs upon application, the patient must wait until the vision clears before driving or using machinery.
ADVERSE EFFECTS
The most frequent adverse reactions to TOPTIC eye drops is localized ocular toxicity and hypersensitivity, including punctate keratitis, eye and lid itching, lid swelling, ocular hyperaemia, conjunctival erythema and lacrimation. These reactions occur in approximately 3% of patients treated with TOPTIC.
A summary of treatment emergent adverse events based on literature and post marketing experience and their estimate of frequencies (very common, common, uncommon, rare, very rare, and not known) in accordance with preferred term and system organ classes (SOC) of any severity are listed below.
Within each frequency-grouping, undesirable effects are presented in decreasing order of seriousness. These adverse reactions were observed following ophthalmic use of Tobramycin Eye Drops:
Immune system disorders:
Uncommon (> 0.1% to 1%): hypersensitivity
Nervous system disorders:
Uncommon (> 0.1% to 1%): headache
Eye disorders:
Common (> 1% to < 10%): ocular discomfort, ocular hyperaemia Uncommon (> 0.1% to 1%): keratitis, corneal abrasion, conjunctival disorder, visual impairment, vision blurred, erythema of eyelid, conjunctival oedema, eyelid oedema, eyelid disorder, eye pain, dry eye, eye discharge, eye pruritus, foreign body sensation in eyes, lacrimation increased
Not known: eye allergy, eye irritation, eyelids pruritus
Skin and subcutaneous tissue disorders:
Uncommon (> 0.1% to 1%): urticaria, dermatitis, madarosis, leukoderma, pruritus, dry skin
Not Known: rash
If topical ocular tobramycin is administered concomitantly with systemic aminoglycoside antibiotics, the possibility of increased systemic toxicity cannot be excluded and care should be taken to monitor the total serum concentration.
Prolonged levels above 12 mcg/mL should be avoided.
DOSAGE AND ADMINISTRATION
TOPTIC Eye Drops
In mild to moderate disease, instill one or two drops into the affected eye(s) every four hours.
In severe infections, instill two drops into the eye(s) hourly until improvement, following which treatment should be reduced prior to discontinuation. The usual duration of treatment is 7 to 10 days.
OVER DOSAGE
Clinically apparent signs and symptoms of TOPTIC overdose are not expected when used as above nor in the event of accidental ingestion of the contents of one bottle or tube. However, excessive local reactions may occur. In such cases treatment should be discontinued and appropriate treatment instituted.
A topical overdose of TOPTIC may be flushed from the eye(s) with lukewarm water.
POISON SCHEDULE OF THE MEDICINE
Prescription only Medicine.
PRESENTATION AND STORAGE CONDITIONS
TOPTIC Eye Drops 5 mL DROP-TAINER dispenser, containing tobramycin 0.3% (3 mg/ml).
USUAL DOSAGE:
Instill one or two drop in the affected eye(s), every four to six hours.
Or as directed by the doctor.
DIRECTIONS FOR USAGE:
- Protect from light, store at 15°C – 25°C.
- Do not freeze. Keep out of the reach of children.
- Do not touch the nozzle tip to any surface as this may contamination the solution.
- Do not use longer than one month after first opening.
WARNING: To be sold on prescription of a registered medical practitioner only.


