

More than 200 Distributors
Around 1,000,000 Outlets
Nationwide Coverage
Somezol
(Esomeprazole)
Brand Name
Somezol
Generic Name
(Esomeprazole)
Therapeutic Segment
Anti-Ulcerant

Scan QR code to open on your mobile device.
Available as
- INJECTION
- SOMEZOL 20MG INJECTION
- SOMEZOL 40MG INJECTION
- CAPSULE
- SOMEZOL 20MG CAPSULE
- SOMEZOL 40MG CAPSULE
PRESCRIBING INFORMATION
Description:
The active ingredient in SOMEZOL I.V. (esomeprazole sodium) for Injection is (S)-5-methoxy-2[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]- 1 H-benzimidazole sodium a compound that inhibits gastric acid secretion. Esomeprazole is the S-isomer of omeprazole, which is a mixture of the S- and R- isomers.Its empirical formula is C17H18N3O3SNa with molecular weight of 367.4 g/mol (sodium salt) and 345.4 g/mol (parent compound). Esomeprazole sodium is very soluble in water and freely soluble in ethanol (95%). The structural formula is:
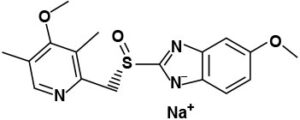
SOMEZOL I.V. for Injection is supplied as a sterile, freeze-dried, white to off-white powder intended for intravenous administration after reconstitution with 0.9% Sodium chloride Injection, USP; Lactated Ringers Injection, USP or 5% Dextrose Injection, USP. SOMEZOL I.V. for Injection contains esomeprazole sodium 21.3mg equivalent to esomeprazole 20mg & 42.5mg equivalent to esomeprazole 40mg. The stability of esomeprazole sodium in aqueous solution is strongly pH dependent. The rate of degradation increases with decreasing pH.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Absorption: Once daily, administration of SOMEZOL I.V infusion of 20mg & 40mg over 30 minutes for five days. The results are shown in the following table:
|
Pharmacokinetic Parameters of SOMEZOL Following I.V. Dosing for 5 days |
||
|
Parameter |
Somezol IV 20mg |
Somezol IV 40mg |
|
AUC (mol*h/L) |
5.11 |
16.21 |
|
Cmax (mmol/L) |
3.86 |
7.51 |
|
t1/2 (h) |
1.05 |
1.41 |
Distribution:
Esomeprazole is 97% bound to plasma proteins. Plasma protein binding is constant over the concentration range of 2-20 mol/L. The apparent volume of distribution at steady state in healthy volunteers is approximately 16 L.
Metabolism:
Esomeprazole is extensively metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The metabolites of esomeprazole lack antisecretory activity. The major part of esomeprazole’s metabolism is dependent upon the CYP2C19 isoenzyme, which forms the hydroxy and desmethyl metabolites. The remaining amount is dependent on CYP3A4 which forms the sulphone metabolite.
Excretion:
Esomeprazole is excreted as metabolites primarily in urine but also in feces. Esomeprazole is completely eliminated from plasma and there is no accumulation during once daily administration. The plasma elimination half-life of intravenous esomeprazole is approximately 1.1 to 1.4 hours and is prolonged with increasing dose of intravenous esomeprazole.
Special Populations:
Investigation of age, gender, race, renal, and hepatic impairment and metabolizer status have been made with oral esomeprazole. The pharmacokinetics of esomeprazole is not expected to be affected differently by intrinsic or extrinsic factors after intravenous administration compared to oral administration. The same recommendations for dose adjustment in special populations are suggested for intravenous esomeprazole as for oral esomeprazole.
Geriatric:
The AUC and Cmax values were slightly higher (25% and 18%, respectively) in the elderly as compared to younger subjects at steady state. Dosage adjustment based on age is not necessary.
Gender:
The AUC and Cmax values were slightly higher (13%) in females than in males at s teady state. Dosage adjustment based on gender is not necessary.
Hepatic Insufficiency:
In patients with mild and moderate hepatic insufficiency, the AUCs were within the range that could be expected in patients with normal liver function. In patients with severe hepatic insufficiency the AUCs were 2 to 3 times higher than in the patients with normal liver function. No dosage adjustment is recommended for patients with mild to moderate hepatic insufficiency . However, in patients with severe hepatic insufficiency a dose of 20 mg once daily should not be exceeded.
Renal Insufficiency:
The pharmacokinetics of esomeprazole in patients with renal impairment are not expected to be altered relative to healthy volunteers as less than 1% of esomeprazole is excreted unchanged in urine.
PHARMACODYNAMICS
Mechanism of Action:
Esomeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+/K+-ATPase in the gastric parietal cell. The S- and R-isomers of omeprazole are protonated and converted in the acidic compartment of the parietal cell forming the active inhibitor, the achiral sulphenamide. By acting specifically on the proton pump, esomeprazole blocks the final step in acid production, thus reducing gastric acidity. This effect is dose-related up to a daily dose of 20 to 40 mg and leads to inhibition of gastric acid secretion.
Therapeutic Indications:
Somezol for injection and infusion is indicated for gastric antisecretory treatment when the oral route is not possible such as, Gastroesophageal reflux disease in patients with Esophagitis or severe symptoms of reflux. Healing of gastric ulcers associated with NSAID therapy Prevention of gastric and duodenal ulcer associated with NSAID therapy, in patients at risk.
DOSAGE AND ADMINISTRATION
SOMEZOL I.V. for Injection should not be administered concomitantly with any other medications through the same intravenous site and or tubing. The intravenous line should always be flushed with either 0.9% Sodium chloride Injection, USP, Lactated Ringers Injection, USP or 5% Dextrose Injection, USP both prior to and after administration of SOMEZOL I.V. for Injection.
Treatment with SOMEZOL I.V. for Injection should be discontinued as soon as the patient is able to resume treatment with SOMEZOL Capsules. Safety and efficacy of SOMEZOL I.V. for Injection as a treatment of GERD patients with a history of erosive esophagitis for more than 10 days have not been demonstrated.
Adult Patients
The recommended adult dose is either 20mg or 40mg SOMEZOL given once daily by intravenous injection (no less than 3 minutes) or intravenous infusion (10 minutes to 30 minutes).
Dosage adjustment is not required in patients with mild to moderate liver impairment (Child Pugh Classes A and B). For patients with severe liver impairment (Child Pugh Class C), a maximum dose of 20 mg once daily of SOMEZOL should not be exceeded [see , CLINICAL PHARMACOLOGY].
Pediatric Patients
The recommended doses for children ages 1 month to 17 years, inclusive, are provided below. Dose should be infused over 10 minutes to 30 minutes.
1 year to 17 years:
- Body weight less than 55 kg: 10 mg
- Body weight 55 kg or greater: 20 mg
1 month to less than 1 year of age: 0.5 mg/kg
Preparations for use
Intravenous Injection (20mg & 40mg) over no less than 3 minutes
The freeze-dried powder should be reconstituted with 5 mL of 0.9% Sodium Chloride Injection, USP. Withdraw 5 mL of the reconstituted solution and administer as an intravenous injection over no less than 3 minutes.
The reconstituted solution should be stored at room temperature up to 30 OC (86OF) and administered within 12 hours after reconstitution. No refrigeration is required.
Intravenous Infusion (20mg & 40mg) over 10 to 30 minutes A solution for intravenous infusion is prepared by first reconstituting the contents of one vial with 5mL of 0.9% Sodium Chloride Injection, USP, Lactated Ringers Injection, USP or 5% Dextrose Injection, USP and further diluting the resulting solution to a final volume of 50 mL. The solution (admixture) should be administered as an intravenous infusion over a period of 10 to 30 minutes.
The admixture should be stored at room temperature up to 30 OC (86OF) and should be administered within the designated time period as listed in the Table below. No refrigeration is required.
|
Diluent |
Administer within |
|
0.9 % Sodium Chloride Injection, USP |
12 hours |
|
Lactated Ringers Injection, USP |
12 hours |
|
5 % Dextrose Injections, USP |
6 hours |
SOMEZOL I.V. for Injection should not be administered concomitantly with any other medications through the same intravenous site and or tubing. The intravenous line should always be flushed with either 0.9% Sodium Chloride Injection, USP, Lactated Ringers Injection, USP or 5% Dextrose Injection, USP both prior to and after administration of SOMEZOL I.V. for Injection.
Reconstitute solution should be inspected visually for particulate matter and discoloration prior to administration, only clear solution should be used.
SIDE EFFECTS
Adverse experiences occurring in >1% of patients treated with intravenous esomeprazole are listed below by body system:
Skin and appendages disorders: pruritus(1.1%).
Central and peripheral nervous system disorders: dizziness (2.5%), headache (10.9%).
Gastrointestinal system disorders: abdominal pain (5.8%), constipation (2.5%), diarrhea (3.9%), dyspepsia (6.4%), flatulence (10.3%), mouth dry (3.9%), nausea (6.4%).
Respiratory System disorders: respiratory infection (1.1%), sinusitis (1.7%).
Body as a whole general disorder: AE associated with test procedure (23.1%), and Application site disorders: application site reaction (1.7%) (including mild focal erythema and pruritus at IV insertion site).
Intravenous treatment with esomeprazole 20mg & 40mg administered as an injection or as an infusion was found to have a safety profile similar to that of oral administration of esomeprazole 20mg & 40mg.
DRUG INTERACTIONS
Coadministration of oral contraceptives, diazepam, phenytoin, or quinidine did not seem to change the pharmacokinetic profile of esomeprazole.
Concomitant administration of esomeprazole and either (nonselective ) or rofecoxib ( selective NSAID) did not identify any clinically relevant changes in the pharmacokinetic profiles of esomeprazole or these NSAIDs.
Esomeprazole inhibits acid secretion. Therefore, esomeprazole may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability (eg, ketoconazole, salts and digoxin).
WARNINGS AND PRECAUTIONS
In the presence of any alarm symptoms (e.g. significant unintentional weight loss, recurrent vomiting, dysphagia haematemesis or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded, as treatment with Somezol IV may alleviate symptoms and delay diagnosis.
PREGNANCY
Teratogenic Effects. Pregnancy Category B
Teratology studies have been performed in rats at oral doses up to 280 mg/kg/day (about 57 times the human dose on a body surface area basis) and in rabbits at oral doses up to 86 mg/kg/day (about 35 times the human dose on a body surface area basis) and have revealed no evidence of impaired fertility or harm to the fetus due to esomeprazole. There are, however, no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers:
It is not known whether Esomeprazole is excreted in human breast milk. No studies in lactating women have been performed. Therefore, Somezol IV should not be used during breast feeding.
Pediatric Use:
Safety and effectiveness in pediatric patients have been established.
Geriatric Use:
No overall differences in safety and efficacy were observed between the elderly and younger individuals.
OVERDOSE
No specific antidote for Esomeprazole is known. Since Esomeprazole is extensively protein bound, it is not expected to be removed by dialysis. In the event of over dosage, treatment should be symptomatic and supportive. As with the management of any overdose, the possibility of multiple drug ingestion should be considered.
CONTRAINDICATIONS
SOMEZOL is contraindicated in patients with known hypersensitivity to any component of the formulation or to substituted benzimidazoles.
HOW SUPPLIED
SOMEZOL I.V. for Injection is supplied as a freeze-dried powder containing 20mg & 40mg of esomeprazole per single-use vial alongwith 0.9% sodium chloride 5ml (Soride).
STORAGE
- Protect from heat, sunlight and moisture, store at 25 oC.
- Keep out of the reach of children.
- Patients and healthcare professionals can also report suspected adverse drug reaction at ade@bosch-pharma.com.
- To be sold on prescription of a registered medical practitioner only.
DESCRIPTION:
The active ingredient in SOMEZOL (Esomeprazole) is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5- dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole-1-yl) magnesium trihydrate, a compound that inhibits gastric acid secretion more effectively than omeprazole.
Esomeprazole is the S-isomer of omeprazole, which is a mixture of the S- and R- isomers. Its empirical formula is (C17H18N3O3S)2Mgx3H2O with molecular weight of 767.2 as a trihydrate and 713.1 on an anhydrous basis. The structural formula is:
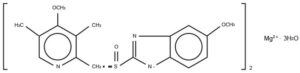
The magnesium salt is a white to slightly colored crystalline powder. It contains 3 moles of water of solvation and is slightly soluble in water.
COMPOSITION:
SOMEZOL Capsules 20mg:
Each capsule contains:
Esomeprazole ….. 20mg as Esomeprazole Magnesium Trihydrate U.S.P. (Enteric Coated Pellets)
(Product Specs.: U.S.P.)
SOMEZOL Capsules 40mg:
Each capsule contains:
Esomeprazole ….. 40mg as Esomeprazole Magnesium Trihydrate U.S.P. (Enteric Coated Pellets)
(Product Specs.: U.S.P.)
CLINICAL PHARMACOLOGY:
Pharmacokinetics
Absorption
SOMEZOL Capsules contain an enteric-coated pellet formulation of Esomeprazole magnesium. After oral administration peak plasma levels (Cmax) occur at approximately 1.5 hours (Tmax). The Cmax increases proportionally when the dose is increased, and there is a three-fold increase in the area under the plasma concentration-time curve (AUC) from 20 to 40 mg. At repeated once daily dosing with 40 mg, the systemic bioavailability is approximately 90% compared to 64% after a single dose of 40mg.
Effect of food: The AUC after administration of a single 40 mg dose of Esomeprazole is decreased by 43-53% after food intake compared to fasting conditions. Esomeprazole should be taken at least one hour before meals. Food delays and decreases the absorption of Esomeprazole, but this does not significantly change its effect on the intra gastric acidity.
Distribution
Esomeprazole is 97% bound to plasma proteins. Plasma protein binding is constant over the concentration range of 2-20 μmol/L. The apparent volume of distribution at steady state in healthy volunteers is approximately 16 L.
Metabolism
Esomeprazole is extensively metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The metabolites of Esomeprazole lack antisecretory activity. The major part of Esomeprazole’s metabolism is dependent upon the CYP2C19 isoenzyme, which forms the hydroxy and desmethyl metabolites. The remaining amount is dependent on CYP3A4 which forms the sulphone metabolite.
Excretion
The plasma elimination half-life of Esomeprazole is approximately 1-1.5 hours. Less than 1% of parent drug is excreted in the urine. Approximately 80% of an oral dose of Esomeprazole is excreted as inactive metabolites in the urine, and the remainder is found as inactive metabolites in the feces.
Special Populations:
Geriatric
The AUC and Cmax values were slightly higher (25% and 18%, respectively) in the elderly as compared to younger subjects at steady state. Dosage adjustment based on age is not necessary.
Pediatric
The pharmacokinetics of Esomeprazole have not been studied in patients < 18 years of age.
Gender
The AUC and Cmax values were slightly higher (13%) in females than in males at steady state. Dosage adjustment based on gender is not necessary.
Hepatic Insufficiency:
In patients with mild and moderate hepatic insufficiency, the AUCs were within the range that could be expected in patients with normal liver function. In patients with severe hepatic insufficiency the AUCs were 2 to 3 times higher than in the patients with normal liver function. No dosage adjustment is recommended for patients with mild to moderate hepatic insufficiency (Child Pugh Classes A and B). However, in patients with severe hepatic insufficiency (Child Pugh Class C) a dose of 20 mg once daily should not be exceeded.
Renal Insufficiency:
The pharmacokinetics of Esomeprazole in patients with renal impairment are not expected to be altered relative to healthy volunteers as less than 1% of Esomeprazole is excreted unchanged in urine.
Pharmacodynamics:
Mechanism of Action
Esomeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+ / K+-ATPase in the gastric parietal cell. The S- and R-isomers of omeprazole are protonated and converted in the acidic compartment of the parietal cell forming the active inhibitor, the achiral sulphenamide. By acting specifically on the proton pump, Esomeprazole blocks the final step in acid production, thus reducing gastric acidity. This effect is dose-related up to a daily dose of 20 to 40 mg and leads to inhibition of gastric acid secretion.
Microbiology:
Esomeprazole magnesium, amoxicillin and clarithromycin triple therapy has been shown to be active against most strains of Helicobacter pylori (H. pylori) in vitro and in clinical infections.
INDICATIONS AND USAGE:
Treatment of Gastroesophageal Reflux Disease (GERD)
Healing of Erosive Esophagitis
SOMEZOL is indicated for the short-term treatment (4 to 8 weeks) in the healing and symptomatic resolution of diagnostically confirmed erosive esophagitis. For those patients who have not healed after 4-8 weeks of treatment, an additional 4-8-week course of SOMEZOL may be considered.
Maintenance of Healing of Erosive Esophagitis
SOMEZOL is indicated to maintain symptom resolution and healing of erosive esophagitis.
Symptomatic Gastroesophageal Reflux Disease
SOMEZOL is indicated for treatment of heartburn and other symptoms associated with GERD.
Risk Reduction of NSAID-Associated Gastric Ulcer
SOMEZOL is indicated for the reduction in the occurrence of gastric ulcers associated with continuous NSAID therapy in patients at risk for developing gastric ulcers. Patients are considered to be at risk due to their age (> 60) and/or documented history of gastric ulcers.
- pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Triple Therapy (SOMEZOL plus amoxicillin and clarithromycin): SOMEZOL, in combination with amoxicillin and clarithromycin, is indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or history of within the past 5 years) to eradicate H. pylori.
Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence. In patients who fail therapy, susceptibility testing should be done. If resistance to clarithromycin is demonstrated or susceptibility testing is not possible, alternative antimicrobial therapy should be instituted.
DOSAGE AND ADMINISTRATION:
The recommended adult dosages are outlined in the table below. SOMEZOL Capsules should be swallowed whole and taken at least one hour before eating.
|
Recommended Adult Dosage Schedule |
||
|
Indication |
Dose |
Frequency |
|
I. Gastroesophageal Reflux Disease |
||
|
Healing of erosive esophagitis |
20mg or 40mg |
Once daily 4 to 8 weeks (an additional 4-8 weeks treatment may be considered if symptoms persist or esophagitis does not heal) |
|
Maintenance of healing erosive esophagitis |
Once daily |
Once daily |
|
Symptomatic gastroesophageal reflux disease without esophagitis |
20mg or 40mg |
Once daily for 4 weeks (an additional 4-8 weeks treatment may be considered if symptoms do not resolve completely) |
|
II. H. Pylori eradication to reduce the risk of duodenal ulcer recurrence Somezol Amoxicillin Clarithromycin |
40mg 1g 500mg |
Once daily for 10 days Twice daily for 10 days Twice daily for 10 days |
Special Populations: Geriatric
No dosage adjustment is necessary.
Renal Insufficiency
No dosage adjustment is necessary.
Hepatic Insufficiency
No dosage adjustment is necessary in patients with mild to moderate liver impairment (Child Pugh Classes A and B). For patients with severe liver impairment (Child Pugh Class C), a dose of 20 mg of SOMEZOL should not be exceeded.
Gender:
No dosage adjustment is necessary
CONTRAINDICATIONS:
SOMEZOL is contraindicated in patients with known hypersensitivity to any component of the formulation or to substituted benzimidazoles.
PRECAUTIONS:
General
In the presence of any alarming symptoms (e.g. significant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded, as treatment with Esomeprazole may alleviate symptoms and delay diagnosis. Patients on long term treatment (particularly those treated for more than a year) should be kept under regular surveillance since the symptomatic response to therapy with Esomeprazole does not preclude the gastric malignancy.
Drug Interactions
Drug interaction studies have shown that Esomeprazole does not have any clinically significant interactions with phenytoin, warfarin, quinidine, clarithromycin or amoxicillin. Coadministration of oral contraceptives, diazepam, phenytoin, or quinidine did not seem to change the pharmacokinetic profile of Esomeprazole. Esomeprazole inhibits gastric acid secretion. Therefore, Esomeprazole may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability (eg. ketoconazole, iron salts and digoxin).
Pregnancy
Teratogenic Effects: Pregnancy Category B – Teratology studies have been performed in rats at oral doses up to 280 mg/kg/day (about 57 times the human dose on a body surface area basis) and in rabbits at oral doses up to 86 mg/kg/day (about 35 times the human dose on a body surface area basis) and have revealed no evidence of impaired fertility or harm to the fetus due to Esomeprazole. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
The excretion of Esomeprazole in milk has not been studied. However, omeprazole concentrations have been measured in breast milk of a woman following oral administration of 20 mg. Because Esomeprazole is likely to be excreted in human milk, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
No overall differences in safety and efficacy were observed between the elderly and younger individuals, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
ADVERSE REACTIONS:
The following adverse drug reactions have been reported during therapy of Esomeprazole. None found to be dose-related
Common: Headache, abdominal pain, diarrhoea, flatulence, nausea/vomiting, constipation
Uncommon: Dermatitis, pruritis, urticaria, dizziness, dry mouth
Rare: Hypersensitivity reactions e.g., angioderma, anaphylactic reaction
OVERDOSAGE:
No specific antidote for Esomeprazole is known. Since Esomeprazole is extensively protein bound, it is not expected to be removed by dialysis. In the event of over dosage, treatment should be symptomatic and supportive.
As with the management of any overdose, the possibility of multiple drug ingestion should be considered.
Shelf Life:
03 Years
HOW SUPPLIED:
SOMEZOL 20mg:
Cold Form & Cold Seal Pack of 14’s Capsules.
SOMEZOL 40mg:
Cold Form & Cold Seal Pack of 14’s Capsules.
STORAGE:
- Protect from heat, sunlight & moisture, store below 30°C.
- The expiration date refers to the product correctly stored at the required condition.
- Patients and healthcare professionals can also report suspected adverse drug reaction at ade@bosch-pharma.com.
- Keep out of the reach of children.
- To be sold on prescription of a registered medical practitioner only.

