

More than 200 Distributors
Around 1,000,000 Outlets
Nationwide Coverage
Maclacin
(Clarithromycin)
Brand Name
Maclacin
Generic Name
(Clarithromycin)
Therapeutic Segment
Antibiotic (Macrolide)
Available as
- SUSPENSION
- MACLACIN 125MG/5ML SUSPENSION
- MACLACIN 250MG/5ML (60ML) SUSPENSION
- TABLET
- MACLACIN 250MG TABLET
- MACLACIN 500MG TABLET
PRESCRIBING INFORMATION
DESCRIPTION
Clarithromycin is a semi-synthetic macrolide antibiotic. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. The structural formula is
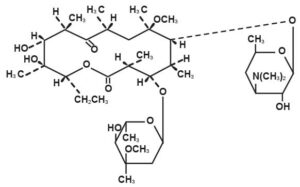
Clarithromycin is a white to off-white crystalline powder. It is soluble in acetone, slightly soluble in methanol, ethanol, and acetonitrile, and practically insoluble in water.
MACLACIN is available as 250mg and 500mg film coated tablets and granules 125mg/5ml and 250mg/5ml for oral suspensions.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Clarithromycin is rapidly absorbed from the gastrointestinal tract after oral administration. The absolute bioavailability of 250mg clarithromycin tablets was approximately 50%. For a single 500mg dose of clarithromycin, food slightly delays the onset of clarithromycin absorption, increasing the peak time from approximately 2 to 2.5 hours. Food also increases the clarithromycin peak plasma concentration by about 24%, but does not affect the extent of clarithromycin bioavailability.
Food does not affect the onset of formation of the antimicrobially active metabolite, 14-OH clarithromycin or its peak plasma concentration but does slightly decrease the extent of metabolite formation, indicated by an 11% decrease in area under the plasma concentration-time curve (AUC). Therefore, Maclacin tablets may be given without regard to food.
In nonfasting healthy human subjects (males and females), peak plasma concentrations were attained within 2 to 3 hours after oral dosing. Steady-state peak plasma clarithromycin concentrations were attained within 3 days and were approximately 1 to 2 μg/mL with a 250mg dose administered every 12 hours and 3 to 4 μg/mL with a 500mg dose administered every 8 to 12 hours. The elimination half-life of clarithromycin was about 3 to 4 hours with 250mg administered every 12 hours but increased to 5 to 7 hours with 500mg administered every 8 to 12 hours. The nonlinearity of clarithromycin pharmacokinetics is slight at the recommended doses of 250mg and 500mg administered every 8 to 12 hours. With a 250mg every 12 hours dosing, the principal metabolite, 14-OH clarithromycin, attains a peak steady-state concentration of about 0.6 μg/mL and has an elimination half-life of 5 to 6 hours. With a 500mg every 8 to 12 hours dosing, the peak steady-state concentration of 14-OH clarithromycin is slightly higher (up to 1 μg/mL), and its elimination half-life is about 7 to 9 hours. With any of these dosing regimens, the steady-state concentration of this metabolite is generally attained within 3 to 4 days.
After a 250mg tablet every 12 hours, approximately 20% of the dose is excreted in the urine as clarithromycin, while after a 500mg tablet every 12 hours, the urinary excretion of clarithromycin is somewhat greater, approximately 30%. In comparison, after an oral dose of 250mg (125mg/5mL) suspension every 12 hours, approximately 40% is excreted in urine as clarithromycin. The renal clearance of clarithromycin is, however, relatively independent of the dose size and approximates the normal glomerular filtration rate. The major metabolite found in urine is 14-OH clarithromycin, which accounts for an additional 10% to 15% of the dose with either a 250mg or a 500mg tablet administered every 12 hours.
Steady-state concentrations of clarithromycin and 14-OH clarithromycin observed following administration of 500mg doses of clarithromycin every 12 hours to adult patients with HIV infection were similar to those observed in healthy volunteers. In adult HIV-infected patients taking 500mg or 1000mg doses of clarithromycin every 12 hours, steady-state clarithromycin Cmax values ranged from 2 to 4μg/mL and 5 to 10μg/mL, respectively. The steady-state concentrations of clarithromycin in subjects with impaired hepatic function did not differ from those in normal subjects; however, the 14-OH clarithromycin concentrations were lower in the hepatically impaired subjects. The decreased formation of 14-OH clarithromycin was at least partially offset by an increase in renal clearance of clarithromycin in the subjects with impaired hepatic function when compared to healthy subjects.
The pharmacokinetics of clarithromycin was also altered in subjects with impaired renal function. Clarithromycin and the 14-OH clarithromycin metabolite distribute readily into body tissues and fluids. There are no data available on cerebrospinal fluid penetration. Because of high intracellular concentrations, tissue concentrations are higher than serum concentrations. Examples of tissue and serum concentrations are presented below.
|
CONCENTRATION (After 250mg q12h) |
||
|
Tissue Type |
Tissue (μg/g) |
Serum (μg/mL) |
|
Tonsil |
1.6 |
0.8 |
|
Lung |
8.8 |
1.7 |
When 250mg doses of clarithromycin as Maclacin suspension were administered to fasting healthy adult subjects, peak plasma concentrations were attained around 3 hours after dosing.
Steady-state peak plasma concentrations were attained in 2 to 3 days and were approximately 2μg/mL for clarithromycin and 0.7μg/mL for 14-OH clarithromycin when 250mg doses of the clarithromycin suspension were administered every 12 hours. Elimination half-life of clarithromycin (3 to 4 hours) and that of 14OH clarithromycin (5 to 7 hours) were similar to those observed at steady state following administration of equivalent doses of Maclacin tablets.
For adult patients, the bioavailability of 10mL of the 125mg/5mL suspension or 10mL of the 250mg/5mL suspension is similar to a 250mg or 500mg tablet, respectively.
In children requiring antibiotic therapy, administration of 7.5mg/kg q12h doses of clarithromycin as the suspension generally resulted in steady-state peak plasma concentrations of 3 to 7μg/mL for clarithromycin and 1 to 2μg/mL for 14-OH clarithromycin.
In HIV-infected children taking 15mg/kg every 12 hours, steady-state clarithromycin peak concentrations generally ranged from 6 to 15μg/mL.
Clarithromycin penetrates into the middle ear fluid of children with secretory otitis media.
|
CONCENTRATION (After 7.5mg/kg 112h for 5 doses) |
||
|
Analyte |
Middle Ear Fluid (μg/mL) |
Serum (μg/mL) |
|
Tonsil |
2.5 |
1.7 |
|
Lung |
1.3 |
0.8 |
In adults given 250mg clarithromycin as suspension (n = 22), food appeared to decrease mean peak plasma clarithromycin concentrations from 1.2 (± 0.4) μg/mL to 1.0 (±0.4) μg/mL and the extent of absorption from 7.2 (±2.5) hr•μg/mL to 6.5 (± 3.7) hr•μg/mL.
When children (n = 10) were administered a single oral dose of 7.5mg/kg suspension, food increased mean peak plasma clarithromycin concentrations from 3.6 (± 1.5) μg/mL to 4.6 (± 2.8) μg/mL and the extent of absorption from 10.0 (± 5.5) hr•μg/mL to 14.2 (± 9.4) hr•μg/mL.
Clarithromycin 500mg every 8 hours was given in combination with omeprazole 40mg daily to healthy adult males. The plasma levels of clarithromycin and 14-hydroxy-clarithromycin were increased by the concomitant administration of omeprazole. For clarithromycin, the mean Cmax was 10% greater, the mean Cmin was 27% greater, and the mean AUC0-8 was 15% greater when clarithromycin was administered with omeprazole than when clarithromycin was administered alone. Similar results were seen for 14-hydroxy-clarithromycin, the mean Cmax was 45% greater, the mean Cmin was 57% greater, and the mean AUC0-8 was 45% greater. Clarithromycin concentrations in the gastric tissue and mucus were also increased by concomitant administration of omeprazole.
|
Clarithromycin Tissue Concentrations 2 hours after Dose (μg/mL)/(μg/g) |
|||||
|
Treatment |
N |
antrum |
Fundus |
N |
mucus |
|
Clarithromycin |
5 |
10.48 ± 2. |
20.81 ± 7.64 |
4 |
4.15 ± 7.74 |
|
Clarithromycin + Omeprazole |
5 |
19.96 ± 4. |
24.25 ± 6.37 |
4 |
39.29 ± 32.79 |
Microbiology
Clarithromycin exerts its antibacterial action by binding to the 50S ribosomal subunit of susceptible microorganisms resulting in inhibition of protein synthesis.
Clarithromycin is active in vitro against a variety of aerobic and anaerobic gram-positive and gram-negative microorganisms as well as most Mycobacterium avium complex (MAC) microorganisms.
Additionally, the 14-OH clarithromycin metabolite also has clinically significant antimicrobial activity.
The 14-OH clarithromycin is twice as active against Haemophilus influenzae microorganisms as the parent compound. However, for Mycobacterium avium complex (MAC) isolates the 14-OH metabolite is 4 to 7 times less active than clarithromycin. The clinical significance of this activity against Mycobacterium avium complex is unknown.
Clarithromycin has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections:
- Aerobic Gram-positive Microorganisms
- Staphylococcus aureus
- Streptococcus pneumoniae
- Streptococcus pyogenes
- Aerobic Gram-negative Microorganisms
- Haemophilus influenzae
- Haemophilus parainfluenzae
- Moraxella catarrhalis
- Other Microorganisms
- Mycoplasma pneumoniae
- Chlamydia pneumoniae (TWAR)
- Mycobacteria
- Mycobacterium avium complex (MAC) consisting of:
- Mycobacterium avium
- Mycobacterium intracellular
Beta-lactamase production should have no effect on clarithromycin activity.
NOTE: Most strains of methicillin-resistant and oxacillin-resistant staphylococci are resistant to clarithromycin. Omeprazole/clarithromycin dual therapy; ranitidine bismuth citrate/clarithromycin dual therapy, omeprazole/ clarithromycin/amoxicillin triple therapy, and lansoprazole/clarithromycin/amoxicillin triple therapy have been shown to be active against most strains of Helicobacter pylori in vitro and in clinical infections The following in vitro data are available, but their clinical significance is unknown. Clarithromycin exhibits in vitro activity against most strains of the following microorganisms; however, the safety and effectiveness of clarithromycin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
- Aerobic Gram-positive Microorganisms
- Streptococcus agalactiae
- Streptococci (Groups C, F, G)
- Viridans group streptococci
- Aerobic Gram-negative Microorganisms
- Bordetella pertussis
- Legionella pneumophila
- Pasteurella multocida
- Anaerobic Gram-positive Microorganisms
- Clostridium perfringens
- Peptococcus niger
- Propionibacterium acnes
- Anaerobic Gram-negative Microorganisms
- Prevotella melaninogenica (formerly Bacteriodes melaninogenicus)
- In vitro Activity of Clarithromycin against Mycobacteria
Clarithromycin has demonstrated in vitro activity against Mycobacterium avium complex (MAC) microorganisms isolated from both AIDS and non-AIDS patients. While gene probe techniques may be used to distinguish M. avium species from M. intracellular, many studies only reported results on M. avium complex (MAC) isolates.
INDICATIONS
Maclacin (clarithromycin tablets, USP) and Maclacin Granules (clarithromycin for oral suspensions, USP) are indicated for the treatment of mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions as listed below:
Adults (Maclacin tablets and Granules for oral suspensions)
Pharyngitis/Tonsillitis due to Streptococcus pyogenes (The usual drug of choice in the treatment and prevention of streptococcal infections and the prophylaxis of rheumatic fever is penicillin administered by either the intramuscular or the oral route. Clarithromycin is generally effective in the eradication of S. pyogenes from the nasopharynx; however, data establishing the efficacy of clarithromycin in the subsequent prevention of rheumatic fever are not available at present).
Acute maxillary sinusitis due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae.
Acute bacterial exacerbation of chronic bronchitis due to Haemophilus influenzae, Haemophilus
parainfluenzae, Moraxella catarrhalis, or Streptococcus pneumoniae.
Community-Acquired Pneumonia due to Haemophilus influenzae, Mycoplasma pneumoniae, Streptococcus pneumoniae, or Chlamydia pneumoniae (TWAR).
Uncomplicated skin and skin structure infections due to Staphylococcus aureus, or Streptococcus pyogenes (Abscesses usually require surgical drainage).
Disseminated mycobacterial infections due to Mycobacterium avium, or Mycobacterium intracellular.
Maclacin (clarithromycin) tablets in combination with amoxicillin and lansoprazole or omeprazole Capsules, as triple therapy, are indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or five-year history of duodenal ulcer) to eradicate H. pylori.
Maclacin tablets in combination with omeprazole capsules or ranitidine bismuth citrate tablets are also indicated for the treatment of patients with an active duodenal ulcer associated with H. pylori infection. However, regimens which contain clarithromycin as the single antimicrobial agent are more likely to be associated with the development of clarithromycin resistance among patients who fail therapy. Clarithromycin-containing regimens should not be used in patients with known or suspected clarithromycin resistant isolates because the efficacy of treatment is reduced in this setting.
In patients who fail therapy, susceptibility testing should be done if possible. If resistance to clarithromycin is demonstrated, a non-clarithromycin-containing therapy is recommended. The eradication of H. pylori has been demonstrated to reduce the risk of duodenal ulcer recurrence.
Children (Maclacin tablets and Granules for oral suspensions)
Pharyngitis/Tonsillitis due to Streptococcus pyogenes.
Community-Acquired Pneumonia due to Mycoplasma pneumoniae, Streptococcus pneumoniae, or Chlamydia pneumoniae (TWAR).
Acute maxillary sinusitis due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae.
Acute otitis media due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae.
Uncomplicated skin and skin structure infections due to Staphylococcus aureus, or Streptococcus pyogenes (Abscesses usually require surgical drainage).
Disseminated mycobacterial infections due to Mycobacterium avium, or Mycobacterium intracellular.
DOSAGE AND ADMINISTRATION
Maclacin (clarithromycin tablets, USP) and Maclacin Granules (clarithromycin for oral suspensions, USP) may be given with or without food.
|
Maclacin ADULT DOSAGE GUIDELINES |
||
|
|
Maclacin Tablets |
|
|
Infection |
Dosage (q12h) |
Duration (days) |
|
Pharyngitis / Tonsillitis due to |
||
|
S. pyogenes |
250mg |
10 |
|
Acute maxillary sinusitis due to |
||
|
H. influenzae, M. catarrhalis, S. pneumoniae |
500mg |
14 |
|
Acute exacerbation of chronic bronchitis due to |
||
|
H. influenzae |
500mg |
7-14 |
|
H. parainfluenzae |
500mg |
7 |
|
M. catarrhalis |
250mg |
7-14 |
|
S. pneumoniae |
250mg |
7-14 |
|
Community-Acquired Pneumonia due to |
||
|
H. influenzae |
250mg |
7-14 |
|
H. parainfluenzae |
— |
— |
|
M. catarrhalis |
— |
— |
|
S. pneumoniae |
250mg |
7 |
|
C. pneumoniae |
250mg |
7-14 |
|
M. pneumoniae |
250mg |
7-14 |
|
Uncomplicated Skin and Skin |
250mg |
7-14 |
|
structure, S. aureus, S. pyogenes |
250mg |
7-14 |
- pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Triple therapy: Maclacin/lansoprazole/amoxicillin
The recommended adult dose is 500mg Maclacin, 30mg lansoprazole, and 1 gram amoxicillin, all given twice daily (q12h) for 10 or 14 days.
Triple therapy: Maclacin/omeprazole/amoxicillin
The recommended adult dose is 500mg Maclacin, 20mg omeprazole, and 1 gram amoxicillin, all given twice daily (q12h) for 10 days. In patients with an ulcer present at the time of initiation of therapy, an additional 18 days of omeprazole 20mg once daily is recommended for ulcer healing and symptom relief.
Dual therapy: Maclacin/omeprazole
The recommended adult dose is 500mg Maclacin given three times daily (q8h) and 40mg omeprazole given once daily (qAM) for 14 days. An additional 14 days of omeprazole 20mg once daily is recommended for ulcer healing and symptom relief.
Dual therapy: Maclacin/ranitidine bismuth citrate
The recommended adult dose is 500mg Maclacin given twice daily (q12h) or three times daily (q8h) and 400mg ranitidine bismuth citrate given twice daily (q12h) for 14 days. An additional 14 days of 400mg twice daily is recommended for ulcer healing and symptom relief. Maclacin and ranitidine bismuth citrate combination therapy is not recommended in patients with creatinine clearance less than 25 mL/min.
Children – The usual recommended daily dosage is 15 mg/kg/day divided q12h for 10 days.
|
PEDIATRIC DOSAGE GUIDELINES |
||||||
|
Based on Body Weight – Dosing Calculated on 7.5mg/kg q12h |
||||||
|
Weight (Kg) |
(lbs) |
Dose (q 12 h) |
125mL |
mg/5 |
250mL |
mg/5 |
|
9 |
9 |
9 |
9 |
q12h |
n/a |
q12h |
|
17 |
17 |
17 |
17 |
q12h |
2.5 mL |
q12h |
|
25 |
25 |
25 |
25 |
q12h |
3.75 mL |
q12h |
|
33 |
33 |
33 |
33 |
q12h |
5 mL |
q12h |
Clarithromycin may be administered without dosage adjustment in the presence of hepatic impairment if there is normal renal function. However, in the presence of severe renal impairment (CRCL<30mL/min), with or without coexisting hepatic impairment, the dose should be halved or the dosing interval doubled.
Mycobacterial infections
Prophylaxis
The recommended dose of Maclacin for the prevention of disseminated Mycobacterium avium disease is 500 mg b.i.d. In children, the recommended dose is 7.5 mg/kg b.i.d. up to 500 mg b.i.d. No studies of clarithromycin for MAC prophylaxis have been performed in pediatric populations and the doses recommended for prophylaxis are derived from MAC treatment studies in children.
Dosing recommendations for children are in the table above.
Treatment
Clarithromycin is recommended as the primary agent for the treatment of disseminated infection due to Mycobacterium avium complex. Clarithromycin should be used in combination with other antimycobacterial drugs that have shown in vitro activity against MAC or clinical benefit in MAC treatment. The recommended dose for mycobacterial infections in adults is 500 mg b.i.d. In children, the recommended dose is 7.5 mg/kg b.i.d. up to 500 mg b.i.d. Dosing recommendations for children are in the table above.
Clarithromycin therapy should continue for life if clinical and mycobacterial improvements are observed.
SIDE EFFECTS
The majority of side effects observed in clinical trials were of a mild and transient nature. Fewer than 3% of adult patients without mycobacterial infections and fewer than 2% of pediatric patients without mycobacterial infections discontinued therapy because of drug-related side effects.
The most frequently reported events in adults taking Maclacin tablets (clarithromycin tablets, USP) were diarrhea (3%), nausea (3%), abnormal taste(3%), dyspepsia (2%), abdominal pain/discomfort (2%), and headache (2%). In pediatric patients, the most frequently reported events were diarrhea (6%), vomiting (6%), abdominal pain(3%), rash (3%), and headache (2%).
Most of these events were described as mild or moderate in severity of the reported adverse events, only 1% was described as severe.
Changes in Laboratory Values
Changes in laboratory values with possible clinical significance were as follows:
Hepatic – elevated SGPT (ALT) < 1%, SGOT (AST) < 1%, GGT < 1%, alkaline phosphatase <1%, LDH < 1%, total bilirubin < 1% Hematologic – decreased WBC < 1%, elevated prothrombin time 1% Renal – elevated BUN 4%, elevated serum creatinine < 1% GGT, alkaline phosphatase, and prothrombin time data are from adult studies only.
DRUG INTERACTIONS
Clarithromycin use in patients who are receiving theophylline may be associated with an increase of serum theophylline concentrations. Monitoring of serum theophylline concentrations should be considered for patients receiving high doses of theophylline or with baseline concentrations in the upper therapeutic range. In two studies in which theophylline was administered with clarithromycin (a theophylline sustained-release formulation was dosed at either 6.5 mg/kg or 12 mg/kg together with 250 or 500 mg q12h clarithromycin), the steady-state levels of Cmax, Cmin, and the area under the serum concentration time curve (AUC) of theophylline increased about 20%.
Concomitant administration of single doses of clarithromycin and carbamazepine has been shown to result in increased plasma concentrations of carbamazepine. Blood level monitoring of carbamazepine may be considered.
When clarithromycin and terfenadine were coadministered, plasma concentrations of the active acid metabolite of terfenadine were three fold higher, on average, than the values observed when terfenadine was administered alone. The pharmacokinetics of clarithromycin and the 14-hydroxy-clarithromycin were not significantly affected by coadministration of terfenadine once clarithromycin reached steady-state conditions. Concomitant administration of clarithromycin with terfenadine is contraindicated.
Clarithromycin 500 mg every 8 hours was given in combination with omeprazole 40 mg daily to healthy adult subjects. The steady-state plasma concentrations of omeprazole were increased (Cmax, AUC0-24, and T1/2 increases of 30%, 89%, and 34%, respectively), by the concomitant administration of clarithromycin. The mean 24-hour gastric pH value was 5.2 when omeprazole was administered alone and 5.7 when co-administered with clarithromycin.
Co-administration of clarithromycin with ranitidine bismuth citrate resulted in increased plasma ranitidine concentrations (57%), increased plasma bismuth trough concentrations (48%), and increased 14-hydroxy-clarithromycin plasma concentrations (31%). These effects are clinically insignificant.
Simultaneous oral administration of Clarithromycin tablets and zidovudine to HIV-infected adult patients resulted in decreased steady-state zidovudine concentrations. When 500mg of clarithromycin were administered twice daily, steady-state zidovudine AUC was reduced by a mean of 12% (n = 4). Individual values ranged from a decrease of 34% to an increase of 14%. Based on limited data in 24 patients, when Clarithromycin tablets were administered two to four hours prior to oral zidovudine, the steady-state zidovudine Cmax was increased by approximately 2-fold, whereas the AUC was unaffected.
Simultaneous administration of Clarithromycin tablets and didanosine to 12 HIV-infected adult patients resulted in no statistically significant change in didanosine pharmacokinetics. Concomitant administration of fluconazole 200mg daily and clarithromycin 500mg twice daily to 21 healthy volunteers led to increases in the mean steady-state clarithromycin Cmin and AUC of 33% and 18%, respectively. Steady-state concentrations of 14-OH clarithromycin were not significantly affected by concomitant administration of fluconazole.
Concomitant administration of clarithromycin and ritonavir (n = 22) resulted in a 77% increase in clarithromycin AUC and a 100% decrease in the AUC of 14-OH clarithromycin. Clarithromycin may be administered without dosage adjustment to patients with normal renal function taking ritonavir. However, for patients with renal impairment, the following dosage adjustments should be considered. For patients with CLCR 30 to 60mL/min, the dose of clarithromycin should be reduced by 50%. For patients with CLCR < 30 mL/min, the dose of clarithromycin should be decreased by 75%.
Spontaneous reports in the post-marketing period suggest that concomitant administration of clarithromycin and oral anticoagulants may potentiate the effects of the oral anticoagulants. Prothrombin times should be carefully monitored while patients are receiving clarithromycin and oral anticoagulants simultaneously.
Elevated digoxin serum concentrations in patients receiving clarithromycin and digoxin concomitantly have also been reported in post-marketing surveillance. Some patients have shown clinical signs consistent with digoxin toxicity, including potentially fatal arrhythmias. Serum digoxin concentrations should be carefully monitored while patients are receiving digoxin and clarithromycin simultaneously.
WARNINGS
CLARITHROMYCIN SHOULD NOT BE USED IN PREGNANT WOMEN EXCEPT IN CLINICAL CIRCUMSTANCES WHERE NO ALTERNATIVE THERAPY IS APPROPRIATE. IF PREGNANCY OCCURS WHILE TAKING THIS DRUG, THE PATIENT SHOULD BE APPRISED OF THE POTENTIAL HAZARD TO THE FETUS. CLARITHROMYCIN HAS DEMONSTRATED ADVERSE EFFECTS OF PREGNANCY OUTCOME AND/OR EMBRYO-FETAL DEVELOPMENT IN MONKEYS, RATS, MICE, AND RABBITS AT DOSES THAT PRODUCED PLASMA LEVELS 2 TO 17 TIMES THE SERUM LEVELS ACHIEVED IN HUMANS TREATED AT THE MAXIMUM RECOMMENDED HUMAN DOSES.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including clarithromycin, and may range in severity from mild to life threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of “antibiotic-associated colitis”.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to discontinuation of the drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
There have been post-marketing reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency.
Deaths have been reported in some such patients.
PRECAUTIONS
General
Clarithromycin is principally excreted via the liver and kidney. Clarithromycin may be administered without dosage adjustment to patients with hepatic impairment and normal renal function. However, in the presence of severe renal impairment with or without coexisting hepatic impairment, decreased dosage or prolonged dosing intervals may be appropriate.
Clarithromycin in combination with ranitidine bismuth citrate therapy is not recommended in patients with creatinine clearance less than 25 mL/min.
Nursing Mothers
It is not known whether clarithromycin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when clarithromycin is administered to a nursing woman
Pediatric Use
Safety and effectiveness of clarithromycin in pediatric patients under 6 months of age have not been established. The safety of clarithromycin has not been studied in MAC patients under the age of 20 months.
Geriatric Use
In a steady-state study in which healthy elderly subjects (age 65 to 81 years old) were given 500mg every 12 hours, the maximum serum concentration and area under the curves of clarithromycin and 14-OH clarithromycin were increased compared to those achieved in healthy young adults.
These changes in pharmacokinetics parallel known age-related decreases in renal function. In clinical trials, elderly patients did not have an increased incidence of adverse events when compared to younger patients. Dosage adjustment should be considered in elderly patients with severe renal impairment.
OVERDOSE
Overdosage of clarithromycin can cause gastrointestinal symptoms such as abdominal pain, vomiting, nausea, and diarrhea.
Adverse reactions accompanying overdosage should be treated by the prompt elimination of unabsorbed drug and supportive measures. As with other macrolides, clarithromycin serum concentration is not expected to be appreciably affected by hemodialysis or peritoneal dialysis.
CONTRAINDICATIONS
Clarithromycin is contraindicated in patients with a known hypersensitivity to clarithromycin, erythromycin, or any of the macrolide antibiotics.
Concomitant administration of clarithromycin and any of the following drugs is contraindicated:
cisapride, pimozide, terfenadine.
HOW SUPPLIED
Maclacin 250mg Tablet: Alu Alu Pack of 10’s Tablets.
Maclacin 500mg Tablet: Alu Alu Pack of 10’s Tablets.
Maclacin 125 mg / 5ml suspension (60 ml) in 90 ml amber glass bottle.
Maclacin 250 mg / 5ml suspension (60 ml) in 90 ml amber glass bottle.
Protect from light & moisture, store below 30oC.
Keep out of the reach of children.
WARNING:
To be sold on prescription of a registered medical practitioner only.


